|
|
 |
BioSense Corporation Home Page
BioSense Corporation is a pre-production stage company with a proprietary medical device technology for self-diagnosis of fertility status by women everywhere (= "can I - or would I - conceive today?"), and for optional use by health providers.
Cervical cancer screening, pelvic inflammatory disease alert, and the management of premenstrual syndrome are some of the other potential medical applications beyond the basic consumer use.
These prospective additional features are important to medicine in general and to the health insurance companies in particular. As the chemicalization and stressfulness of our life increase, proper management of medical drug prescribing becomes not only more and more difficult but also more and more critical. For example, the management of PMS versus depression is a big issue because the prevalence of depression is as high as one out of three women. It is only likely to increase, as severe depression is expected to be the second greatest cause of disability within two decades.
The other market application of the BioSense (TM) technologies is in agriculture, for breeding cows and other animals. The biosensor innovation leads to the capability to track folliculogenesis, which makes for much better determination of the correct breeding time. Pre-selection of fetal sex may be attempted with the technology.
Because of the two very different market applications of the technology, BioSense is considered to be a holding company over two operating companies, each serving the respective marketplace.
The thinking behind the concept of BioSense (TM)
A society-wide increase in the use of effective Natural Family Planning/Fertility Awareness tools for personal management of reproduction and of family planning could have far-reaching consequences for quality of life, including population growth and divorce trends.
The applicability of the technology in the very different market of agribusiness reduces the risk while potentially multiplying the return on investment. At the same time, it contributes fundamentally to economic progress.
Studies in a number of countries indicate that fertility awareness or natural family planning can be highly satisfactory birth control and family planning methods. This is so particularly for women and couples about 30 plus years old, and those who can practice the methods without teacher assistance.
Our BioMeter is designed to make that possible for any couple.
The same population of people also has problems with impaired fertility, the solutions to which are also aided by tools for personal fertility monitoring. This is one of the areas where the Internet connection between patient and physician has potential to significantly reduce the cost of healthcare.
The reproductive cycle is of primary importance in women's healthcare. Health of one half of humanity revolves around the menstrual cycle. We have shown that the BioMeter is a fundamental improvement over other fertility-monitoring techniques.
We have shown that the fertile window published by other groups is bound to be in error, due to the absence of definitive determination of ovulation in the published studies. In those studies, ovulation was merely estimated by means of various surrogate indicators of fertility.
The announcement of a 6-day fertile window (down from 10 days or more) caused a sensation in the US media, reflecting the consumers' great interest in the subject. Public health specialists confirm the interest and call for better resources.
We have shown that good evidence in the studies exists for a three or four-day period of high probability of conception (+/-1 day) but that the position of the fertile period with respect to ovulation is currently in doubt. With an unequivocal detection of the ovulation event by the company's BioMeter technology, the timing of the fertile window should be equally unequivocal.
The commercial products that claim being useful for personal fertility monitoring have not contributed to the definitive determination of the fertile window, and have not been approved by the FDA for birth control use. We have presented evidence in support of our contention that, for the determination of the fertile window, the other techniques work with inadequate indicators of fertility status. The BioMeter technology promises to offer the definitive solution.
|
What is needed to get BioSense where we want to be?
The proceeds of financing will be used for product manufacturing, statistical clinical trials in multi-center studies, further development of the products, further prosecution of the patents, and operating expenses. The operation plan Gannt chart describes in detail the tasks and their estimated costs to achieve product launches into four different market segments, with the first product introduction projected aggressively within 15 months (+ or - 3).
Capital requirements have been planned to cover top-notch hardware and software engineering, field testing, and marketing. The productization and product introduction will occur in a systematic manner, and the regulatory barrier need not be feared - after all, a prototype of the technology has already received the FDA 510(k). The exit for investors is expected within 2 to 5 years (via an IPO or an M&A) and, with properly executed marketing, it should occur sooner rather than later.
What is the estimated cost and time to complete?
The estimated cost of the 5-year plan is rounded up to $ 10 million USD. With an earlier exit contemplated for the seed investors, the estimated time to complete the whole business plan is 5 years because it is only in year 5 that we have projected the introduction of the lower-priced birth-control device, after the introductions of the product into the clinical market in year 2 and into the self-help conception (reduced fertility) market in year 3. The earlier product price will be circa 3-4 times higher than the price of the last-introduced birth-control product, which is conceptualized as every woman's friendly companion that should be priced so low as to be easily bought on impulse.
The profit margin, inherent in the product, is so high that we can contemplate the price of $ 30 for the birth-control product and still be profitable. The unit cost has been estimated to fall below $ 10 at not too high quantities, and it will be below $ 5 when quantities go really up, upon the introduction of the birth-control model.
The exit for investors is planned for year 3 or 4, that is before the launch of the inexpensive product version, as the costs of marketing will rationalize the IPO or M&A.
Net profit is projected at $ 11 million in year 3 and at $ 39 million in year 4, with net earnings per share at $1.38 and $ 4.88, respectively, in those years. These projections are based on fairly conservative revenue assumptions. Actually, we have and believe in the feasibility of more aggressive projections.
For more details, click either on About BioSense, or on More detailed discussion of BioSense.
|
Safe-Harbor Statement under the Private Securities Litigation Reform Act of 1995
The communications about BioSense Corporation contain forward-looking information within the meaning of Section 21E of the Securities Exchange Act of 1934. These include and/or may include statements regarding the proposed financing's impact on the Company's ability to execute its business plan, the Company's ability to complete the registration process, as well as other statements that may include the words "projected", "estimated", "expects", "anticipates", or similar expressions. Such forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause the actual results, performance or achievements of BioSense to differ materially from those implied or expressed by such forward-looking statements.
|
ADVANTAGES OF THE BIOSENSE FERTILITY MONITOR
The BioSense fertility monitor is the definitive solution to the problem of detecting the brief fertility window, a problem that has eluded solution ever since the limited periodic recurrence of female fertility has been understood.
Previous diagnostic approaches have failed to provide a reliable indication of fertility because they monitor parameters that are indirect, and more or less physiologically remote with respect to the actual fertility status. This is the case with techniques that monitor the basal body temperature as well as those that monitor electrolytes or marker metabolites in body fluids. The flaw plagues the various conductivity probes for saliva and vaginal mucus as well as the devices that detect sex hormone metabolites in the woman's urine.
It is important to realize that even the sex hormone metabolites are not reliable indicators of fertility because they do not signify the actual fertility status. The sex hormones (including estrogen and the LH) are merely input signals into the mechanism of fertility, reflecting the interactions between the brain and the ovaries of the female but NOT the actual status of the tissues in which fertilization occurs.
Unlike the other techniques, the BioSense monitor interrogates the fertility status of the tissues directly, and it does so in a very simple-to-use manner.
This is a quick summary of the BioMeter paper (part 2), which compares the performance of the BioSense monitor with other fertility diagnosis products, and explains its advantages in detail. It is shown how the BioSense monitor recognizes "challenged" menstrual cycles, in which the synchronization between the brain and the ovarian events has gone awry, and how the monitor nevertheless detects the respective markers, critical for reproductive management.
This capability, resulting from the independent detection of ovulation and of the predictive signals, is unprecedented in the other techniques of fertility status diagnosis. In those older techniques, the hormonal input signals are used as both the predictors and the presumed indicators of the ovulation event, which results in an unacceptable definition of the fertile window.
For that reason, the older techniques cannot be approved for birth control purposes.
The BioSense monitor does not suffer from this fundamental limitation. The BioSense monitor predicts and then detects ovulation even when the menstrual period changes from one cycle to the next. Since the cycle length variability is the rule rather than an exception, the BioSense technology cab be the definitive solution to fertility awareness and natural family planning.
In addition, the BioSense monitor optionally provides important diagnostic information to the physicians, by means of the internally stored menstrual cyclic data, available for an optional download.
Send email
INTERESTED IN MORE DETAILS?
For further information, click on the BioMeter Paper links, Parts 1 and 2, on the left margin (scroll up to them).
|
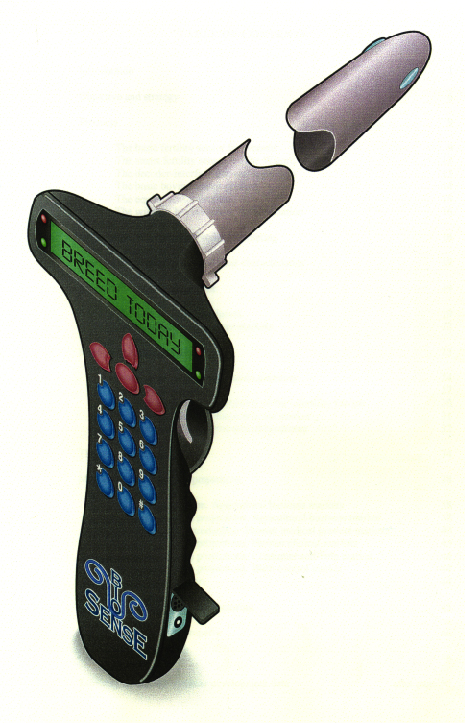
The picture above is an industrial designer's representation of the company's BioMeter (TM) for agricultural uses with small herds and/or with individual prized animals. Large-herd operations will be offered a telemetric version, permanently worn by the animals and obviating the need for manual probing.
The picture at top left is an industrial designer's representation of the miniaturized prototype of BioSense Corporation's Ovulona (TM) device for use by women.
COPYRIGHT NOTICE
This web site, like all web pages, is copyright protected. No license for use of any of the materials published on this web site should be assumed as implied. Nothing stated on any page of this web site should be interpreted as a permission to use these materials. Any use that generates income or interferes with the copyright owner's income or potential income is strictly prohibited.
Send an email
|
|
|


